29, Nov 2023
PEM Fuel Cells: Theory And Practice
PEM Fuel Cells: Theory and Practice
Related Articles: PEM Fuel Cells: Theory and Practice
- 2025 NFL Draft Picks By Team: A Comprehensive Overview
- The Greatest Quarterback Prospects Of All Time: A Comprehensive Analysis
- Wednesday Season 2: A Thrilling And Enchanting Return To Nevermore
- NBA All-Star Game 2025: A Showcase Of Elite Basketball Talent
- Las Vegas To Host 2025 Pro Bowl: A City Of Lights And Excitement
Introduction
With great pleasure, we will explore the intriguing topic related to PEM Fuel Cells: Theory and Practice. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Video about PEM Fuel Cells: Theory and Practice
PEM Fuel Cells: Theory and Practice

Introduction
Proton exchange membrane fuel cells (PEMFCs) are a type of electrochemical cell that generates electricity through the reaction of hydrogen and oxygen. They are a promising technology for clean and efficient power generation, with applications in transportation, stationary power, and portable devices.
Theory of Operation
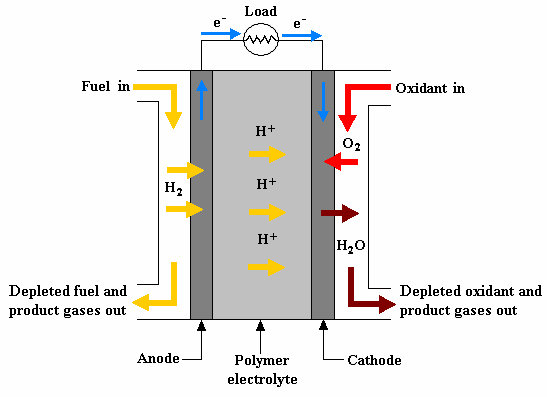
PEMFCs operate on the principle of electrochemistry. Hydrogen gas is fed into the anode of the cell, where it is oxidized to form protons (H+) and electrons. The electrons are conducted through an external circuit, generating an electrical current. The protons pass through the proton exchange membrane (PEM) to the cathode, where they react with oxygen to form water.
The overall reaction in a PEMFC is:
2H2 + O2 → 2H2O + electrical energyThe PEM is a key component of the fuel cell. It allows protons to pass through while blocking electrons, creating a separation between the anode and cathode reactions. The PEM is typically made of a polymer such as Nafion.
Cell Components
A PEMFC consists of several key components:
- Anode: The anode is the negative electrode of the cell. It is made of a porous material, such as carbon paper, that allows hydrogen gas to diffuse into the cell.
- Cathode: The cathode is the positive electrode of the cell. It is made of a porous material, such as carbon paper, that allows oxygen gas to diffuse into the cell.
- Proton exchange membrane (PEM): The PEM is a thin, solid polymer that separates the anode and cathode. It allows protons to pass through while blocking electrons.
- Gas diffusion layers (GDLs): The GDLs are porous layers that facilitate the transport of gases to and from the anode and cathode.
- Catalyst layers: The catalyst layers are thin layers of platinum or platinum alloys that are deposited on the anode and cathode. They catalyze the electrochemical reactions that occur in the cell.
Performance Characteristics
The performance of a PEMFC is determined by several factors, including:
- Cell voltage: The cell voltage is the voltage generated by the cell under operating conditions. It is typically around 0.7 V per cell.
- Current density: The current density is the amount of current that flows through the cell per unit area. It is typically expressed in amperes per square centimeter (A/cm2).
- Power density: The power density is the amount of power generated by the cell per unit area. It is typically expressed in watts per square centimeter (W/cm2).
- Efficiency: The efficiency of a PEMFC is the ratio of the electrical energy generated by the cell to the chemical energy of the hydrogen and oxygen reactants. It is typically around 50-60%.
Applications
PEMFCs have a wide range of potential applications, including:
- Transportation: PEMFCs are used in fuel cell vehicles, which offer zero-emission transportation.
- Stationary power: PEMFCs can be used to generate electricity for buildings, hospitals, and other facilities.
- Portable devices: PEMFCs can be used to power portable devices, such as laptops, cell phones, and drones.
Challenges and Future Directions
PEMFCs are a promising technology, but they face several challenges:
- Cost: PEMFCs are still relatively expensive to produce.
- Durability: PEMFCs can be degraded by impurities in the hydrogen and oxygen reactants.
- Hydrogen infrastructure: The widespread adoption of PEMFCs requires a reliable and affordable hydrogen infrastructure.
Research and development efforts are ongoing to address these challenges. Advances in materials science, manufacturing techniques, and system design are expected to improve the performance, durability, and cost of PEMFCs.
Conclusion
PEMFCs are a promising technology for clean and efficient power generation. They have a wide range of potential applications, but they face several challenges. Ongoing research and development efforts are expected to overcome these challenges and enable the widespread adoption of PEMFCs.







Closure
Thus, we hope this article has provided valuable insights into PEM Fuel Cells: Theory and Practice. We hope you find this article informative and beneficial. See you in our next article!
- 0
- By admin